ION-EXCHANGE CHROMATOGRAPHY
1. THEORETICAL PRINCIPLES
A) Types:
There are two types of ion-exchangers:
cation and anion, in the form of spherical beads called resins. The first
exchange cations by cations and the second anions by anions:
R--H+ +
NaCl ![]() R--Na+ +
HCl,
R--Na+ +
HCl,
R+-OH- + NaCl
![]() R+-Cl- +
NaOH.
R+-Cl- +
NaOH.
The
widely used ion-exchange resins are composed of crosslinked sulphonated
polystyrene with a sulphonic acid group (-SO3H) as the functional
cation-exchange group, or a tetraalkylammonium hydroxide (-CH2-N(CH3)3.OH) as the functional
anion-exchange group (Fig. 19).
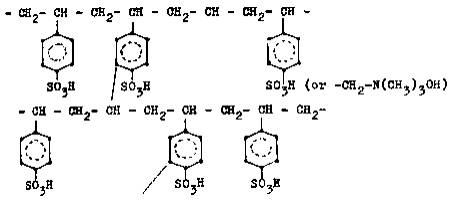
Fig.
19: Crosslinked sulphonated polystyrene.
Since these two groups are strong electrolytes, their resins are
known as strong cation or strong anion-exchangers respectively. Examples of the
different types of resins are given in the following table:
|
Type |
Functional group |
Working pH |
Trade name |
|
Strong cation |
Sulphonic acid |
0 - 14 |
Amberlite IR-120, Dowex 50 |
|
Weak cation |
Carboxylic on polymethacrylate |
5 - 14 |
Amberlite IRC-50 |
|
Strong anion |
Quaternary ammonium |
0 - 12 |
Amberlite IRA-400, Dowex 1. |
|
Weak anion |
Polyamine (2ry or 3ry) on polystyrene or phenylformaldehyde. |
0 - 9 |
Amberlite IR-45, Dowex 3 |
The useful pH ranges are significant. Below pH 5, the weak acid
resins are so slightly dissociated so that cation exchange becomes negligible.
The converse is true for weakly basic types above pH 9.
B) Selectivity coefficient:
Since ion-exchange reactions are reversible, the law of mass
action holds:
2 (RSO3)--K+ +
Ca2+
![]() [(RSO3)-]2-Ca2+ + K+ ;
[(RSO3)-]2-Ca2+ + K+ ;
and:
E
(Ca/K) = [(RSO3)2Ca]
. [K+]2 / [RSO3K]2 . [Ca2+]
Where E (Ca/K) is the selectivity coefficient.
In spite of the
variable nature of selectivity coefficients, it is possible to arrange a series
of ions in the order of their increasing selectivity coefficients for a given
resin, all with respect to a common standard ion. For example, for univalent
cations on sulphonate resins, the order is:
Li+,
H+, Na+, NH4+, K+, Rb+,
Cs+, Ag+, Tl+ ;
That
is Li+ is held least strongly on the resin.
C) Equivalence:
The ion-exchange process occurs in equivalent amounts. When the
potassium form of Dowex 50 (RSO3)-K+ is treated
with a dilute solution of calcium nitrate, the quantity of potassium ions
released from the resin is equivalent to the quantity of calcium ions absorbed.
D) Swelling:
Because the concentration of the internal solution exceeds that of
the external solution, osmotic forces tend to drive water into the resin, thus
causing it to swell. Therefore, the resin must be made fully swollen by
immersing in water before packing to avoid shattering of the column.
E) Regeneration:
To reuse the resin, the absorbed ions have to be removed by
regeneration whereby it is converted to an exchangeable form. This is achieved
by treating the resin with a corresponding acid or alkali (strong acid for
strong cation-exchanger and so on). The form produced is the hydrogen or hydroxyl
respectively. For conversion of the resin from a univalent ion form to a
polyvalent ion form, a more concentrated solution of the former is required
than for the reverse.
2. ANALYSIS
It is common practice in ion-exchange chromatography to collect
and analyse large number of small fractions, generally of equal volumes. The
effective time of analysis can be decreased with the aid of an automatic
fraction collector which is rotated intermittently by the action of a timing,
drop-counting, or weight-actuated device. The most widely used technique for
continuously recording the process of chromatographic separations have been
based on conductivity, pH, radioactivity, refractive index, light absorption
and polarographic measurements.
3. CHROMATOGRAPHIC APPLICATIONS
A) Separation of ion:
Ion-exchange resins are used in the separation of simple ions or
mixtures of them. Fig. 20 shows the complete separation of Na+ and K+
ions as an example. Quantitative analysis was made by titration of the corresponding
Cl- ion by the Mohre method.
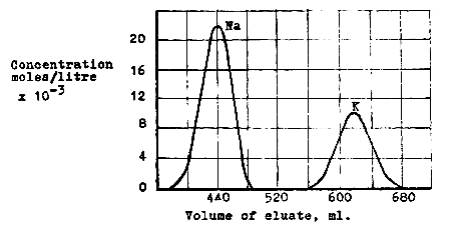
Fig. 20: Ion-exchange separation of sodium and
potassium on a cation-exchange resin, Dowex-50, eluted with 0.7 F-HCl.
B) Removal of interfering ions:
Cations such as Na+, (NH4)+ and
Fe3+ co-precipitate with BaSO4 in gravimetry, and can be
removed by passing the solution through a H+ form cation exchange
resin instead of the other laborious methods.
C) Ion-exclusion:
A mixture of an electrolyte and
non-electrolyte is resolved by passing it over an ion-exchange resin, the
electrolyte emerges first.
D) Salting out:
Mixtures of organic compounds that cannot be separated by elution
with water can often be easily separated by elution with a fairly concentrated
salt solution, hence the nomenclature “salting out”. For example, no separation
is achieved by eluting a mixture of diethylene glycol and dipropylene glycol
through a 70 cm column of Dowex 1-x8 (sulphate form). However, a quantitative
separation is accomplished through a 10 cm column of the same resin by elution
with 3 M-ammonium sulphate.
4. NON-CHROMATOGRAPHIC APPLICATIONS
A) Deionized water:
Removal of ions from water is accomplished by passing it through a
hydrogen form cation-exchanger then a hydroxyl type, or through a mixed bed of
cation-anion resins. Cations are exchanged by hydrogen ions and anions by
hydroxyls, producing water instead of salts. The principle is utilized in the
production of very pure water (conductivity water) for research work, medical
and pharmaceutical purposes, or on the industrial scale. Such water
purification systems may incorporate pre-distillation units to get rid of the
greatest part of salts before inter into the ion-exchangers, whereby the
exhaustion time of resins is increased. Fig. 21 shows a semi-automatic water
purification system involving distillation and deionisation.
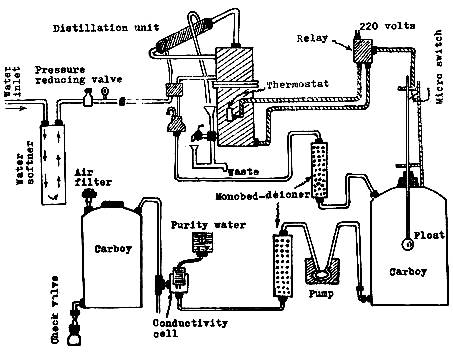
Fig. 21: Apparatus for preparation of highly purified water
for trace analysis.
B) Preparations:
Carbonate free solutions of sodium or potassium hydroxide are
prepared by ion-exchange with the advantage of yielding directly a standard
solution.
C) Concentration of traces:
The ions to be concentrated from large volumes are passed through
a resin and eluted by a small volume of a proper eluent.